Cannabis as medicine, while unregulated
A few months ago, I planned an outing to the Trans-Allegheny Lunatic Asylum in West Virginia. I know it is an odd place to visit but I enjoy all types of history, including medically-related. The facility opened in 1864 and reached its peak in the 1950’s, housing 2,400 patients. Changes in the treatment of mental illness and the facilities physical deterioration led to its closure in 1994. In this one facility I was able to experience first-hand, 130 years of approaches to the treatment of mental illness.
One of the most interesting rooms in this massive facility was the Apothecary. Here, medicines were formulated and dispensed. While the practice of psychopharmacology is considered a relatively modern phenomenon, it is clear that this is not the case. I witnessed compounds that appeared to date back to the early 1900s.
I was wondering what compounds were in use to treat patients. There was a compound manufactured by Parke Davis which contained iron, arsenic, and strychnine, chocolate coated too. For very valid reasons, that combination fell out of favor.
Then I saw another glass flask which was in fact more germane to the modern era. This one from 1906 had cannabis indica as its main ingredient. Clearly, marijuana had a role in treatment of patients over 100 years ago. Why has it taken so much time before an FDA-approved cannabis-based therapy was available? I began to wonder how cannabis and its function in medical practice, has evolved over time.
Cannabis as illegal
In the U.S., cannabis was widely utilized as an over the counter medicine during the 19th and early 20th centuries, described in the United States Pharmacopoeia for the first time in 1850. Then in 1937 with the passage of the Marihuana Tax Act, a federal restriction was placed on cannabis use and sale. Subsequent to this event, cannabis was dropped from the United States Pharmacopoeia in 1942, with legal penalties for its possession increasing in 1951 and 1956 with the enactment of the Boggs and Narcotic Control Acts, respectively, and prohibition under federal law occurring with the Controlled Substances Act of 1970. These legislative actions posed significant constraints on cannabis research by restricting procurement of the substance for academic purposes. In the present day, the Drug Enforcement Agency (DEA) website still labels cannabis a Schedule I drug which is defined as having “no currently accepted medical use and a high potential for abuse.”
Cannabis as an FDA-approved therapy
That said, over 28 states, the District of Columbia, Guam and Puerto Rico have taken their own initiative and have passed their own laws allowing for varying levels of cannabis use. The majority limit this to medicinal cannabis used to treat a range of symptoms. The most common conditions include symptoms of cancer, glaucoma, human immunodeficiency virus, and multiple sclerosis. Recreational use is also legalized in eight states, CO, WA, OR, CA, NV, AK, MA, ME and more to soon follow.
The majority of states are treating serious medical conditions with cannabis and a smaller number are permitted recreational use. I was clearly interested what is happening at the Federal Level.
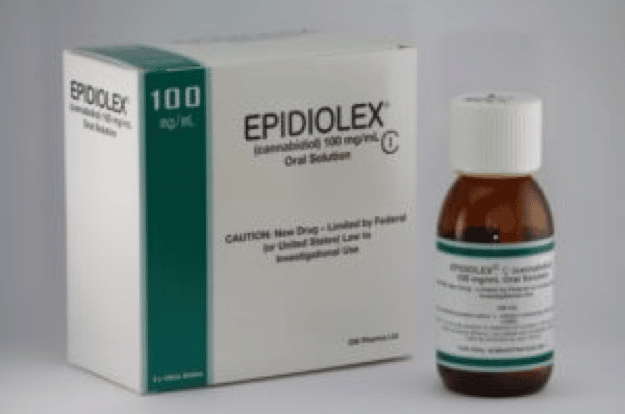
On June 25th, 2018 GW Pharmaceuticals plc, announced that the U.S. FDA approvedEpidiolex® (cannabidiol) oral solution for the treatment of seizures associated with Lennox-Gastaut syndrome (LGS) or Dravet syndrome in patients two years of age or older. The company noted that Epidiolex is the first prescription pharmaceutical formulation of highly-purified, plant-derived cannabidiol (CBD), a cannabinoid lacking the high associated with marijuana, and the first in a new category of anti-epileptic drugs. On June 27th, Revive Therapeutics announced that they have been granted Orphan Drug Designation for cannabidiol in the treatment of autoimmune hepatitis (AIH), a rare disease that causes inflammation of the liver.
There is a clear lack of consistency at the state and federal level concerning cannabis, particularly as a medical therapy. Over a hundred years ago it was legal and nationally available. Then it was completely banned. The majority of states and territories currently allow it for treating severe medical conditions. Eight states allow for recreational cannabis use. The Federal government after rigorous scientific testing approved GW Pharmaceuticals’ product and granted orphan status to Revive Therapeutics’. All the while the DEA maintains cannabis as a Schedule I with “no benefit”. Maybe someday this will get resolved but you got to wonder. Is it the high cannabis can provide preventing the science?
- Market Access: The Latest Hurdle for Treating Alzheimer’s and Dementia - June 14, 2023
- Rare Disease Outreach a Missed Opportunity - November 7, 2022
- So You Read Our Previous Post on Biomarkers? - August 1, 2022