
Every life science product exists within an ecosystem comprised of key stakeholders. Understanding and addressing the needs of these stakeholders demands an integrated approach.
To meet this requirement, Snowfish has developed solutions to help aid therapy interest and uptake by optimizing engagement with three main groups: patients, clinicians, and medical/advocacy organizations on a global basis.
We have been in the business long enough to remember when certain conditions were not widely recognized. Three diseases come to mind: erectile dysfunction (ED), dry eye disease, and myopia in children.
Erectile Dysfunction
While almost everyone is familiar with therapies for ED and dry eye, very few are likely aware that there are ways to slow progression of childhood myopia. This is not due to the latter being any less relevant than the former, rather myopia management strategies have not been around as long.
The current first-line treatment for ED, the oral PDE5 inhibitor, was introduced by Pfizer in 1999. Before Pfizer’s “blue pill,” men suffered in silence. It was an overall embarrassing topic to discuss and the condition was something often associated with aging. In fact, ED really did not have a name.
Billions of dollars were directed towards building awareness among patients, clinicians, and organizations and thus facilitated the conversation. This awareness campaign involved how a clinician should broach the topic of ED with the goal of making both the patient and clinician more open to discuss the disease and the potential treatments.
Dry Eye Disease
Dry eye disease was considered an inconvenience and symptom as opposed to a disease. There was little focus on its long-term implications. The term “dry eye” was first mentioned in the literature back in 1975 by Frank Holly. He concluded that mucin affects tear film formation and stability.
Throughout the later part of the past century eye care professionals (ECPs), which include ophthalmologists and optometrists, would offer moisturizing eye drops since no disease moderating treatment was readily available.
It essentially took 28 years for a Restasis which increases tear production, to be approved.
Still even in 2003 dry eye was not yet defined as a disease but still a symptom with no underlying condition. Below is a brief timeline of how the American Academy of Ophthalmology (AAO) definition of “dry eye” has evolved from a symptom to something that needs to be addressed through treatment:
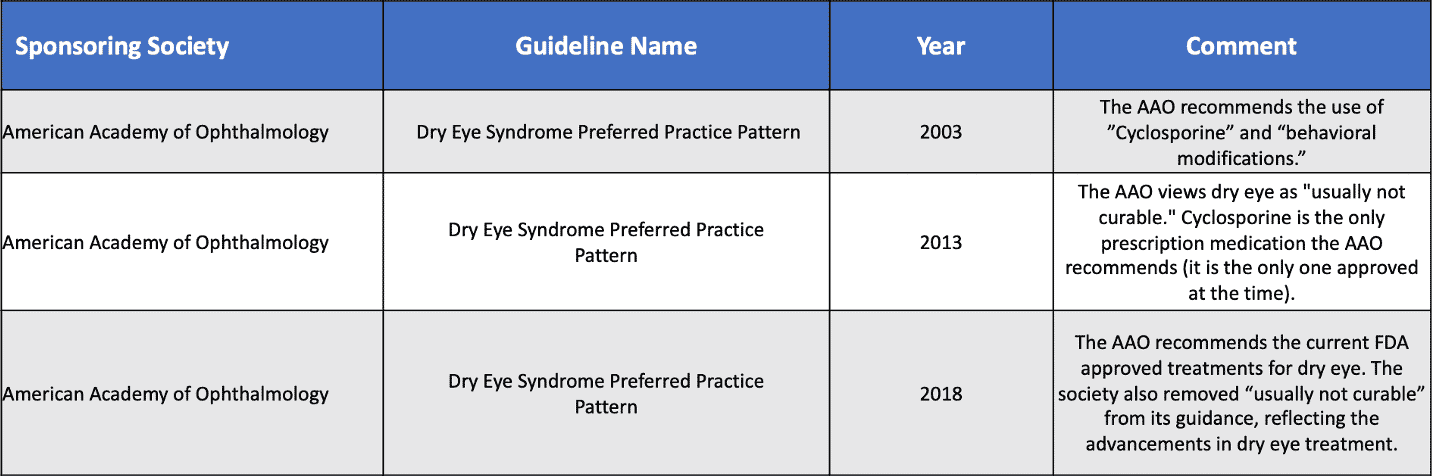
By 2016, Xiidra was approved to treat the symptoms of dry eye disease.
Through the decades it took for dry eye to move from a symptom to a disease in and of itself, other stakeholders were working tirelessly.
For example, back in 2007, Tear Film & Ocular Surface Society’s first International Dry Eye Workshop (DEWS I) acknowledged dry eye as a “disease.”
In fact, DEWS II came out with a stronger statement ten years later in 2017, “Dry eye is a multifactorial disease of the ocular surface characterized by a loss of homeostasis of the tear film, and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation, and damage.”
Despite this progress, far less than 50% of eligible patients are being treated for dry eyes, therefore more work still needs to be done. It is clear that it takes a tremendous amount of effort with patients, clinicians, and medical associations to start to change the treatment paradigm and raise awareness.
Myopia Management in Children
Today, many in the general public are not aware that there are effective interventions to slow the progression of myopia in children. In fact, before we started supporting a company at the forefront of these new technologies, we too were unaware of the availability of such therapies.
Most ECPs still only offer corrective lenses to treat myopia and don’t even discuss options that fall within the the category of “myopia management” and are essentially disease modifying. Low single digits of ECPs are offering myopia management in children.
As a parent I wish I was offered the choice for my daughter to slow her progression the first time she was diagnosed with myopia. At the moment, there is no national or global clinical practice guidelines for treating myopia even though products are approved in multiple countries to treat myopia in children.
Science is ahead of the medical and advocacy organizations; much work needs to be done.
Snowfish has worked for over two decades to connect the dots within the three important stakeholder categories: patients, clinicians and organizations.
It is critical to identify patients that could benefit from new treatments. This involves defining patient attributes and how they receive information from their own influencers including advocacy and support groups.
All of the various inputs are cataloged so our clients can improve the outreach efforts. At times this involves hundreds of unique inputs that allow for micro targeting.
Data are married up to the clinicians that are at the forefront of the disease. By identifying the leading key opinion leader (KOL) and the Centers of Excellence (CoEs), we empower companies with cutting-edge therapies to connect with patients and clinicians.
We also identify KOLs working with association and advocacy groups so that engagement can be leveraged through those channels.
Professional associations promulgate clinical practice guidelines which are foundational to the practice of medicine.
Snowfish will analyze dozens of associations based on multiple factors such as their views regarding a given treatment, key people, publication and social media, constituents, relationships with industry, and collaboration with other organizations. This enables our clients to develop a winning engagement plan with a select group.
The life science industry is constantly evolving. It takes an ongoing and sustain engagement strategy with patients, clinicians, and organizations to make an impact.
David Fishman is the President and Founder of Snowfish. Snowfish has worked with leading companies providing such insight for companies such as Sanofi, AstraZeneca, CooperVision, and Medtronic.
- Market Access: The Latest Hurdle for Treating Alzheimer’s and Dementia - June 14, 2023
- Rare Disease Outreach a Missed Opportunity - November 7, 2022
- So You Read Our Previous Post on Biomarkers? - August 1, 2022



